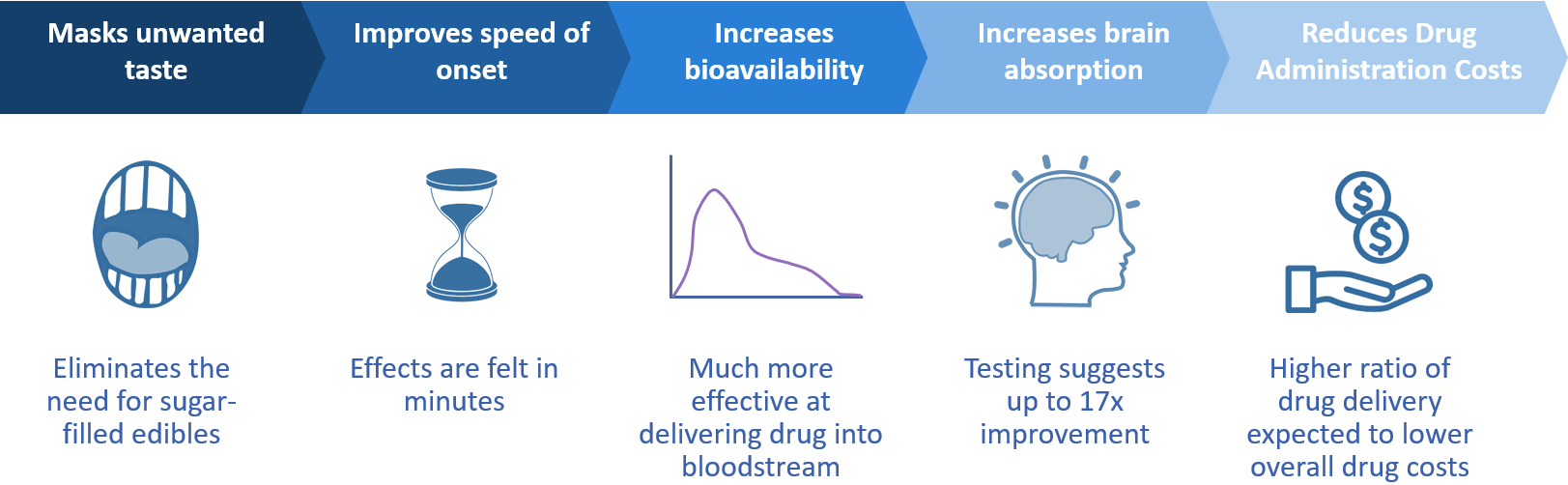
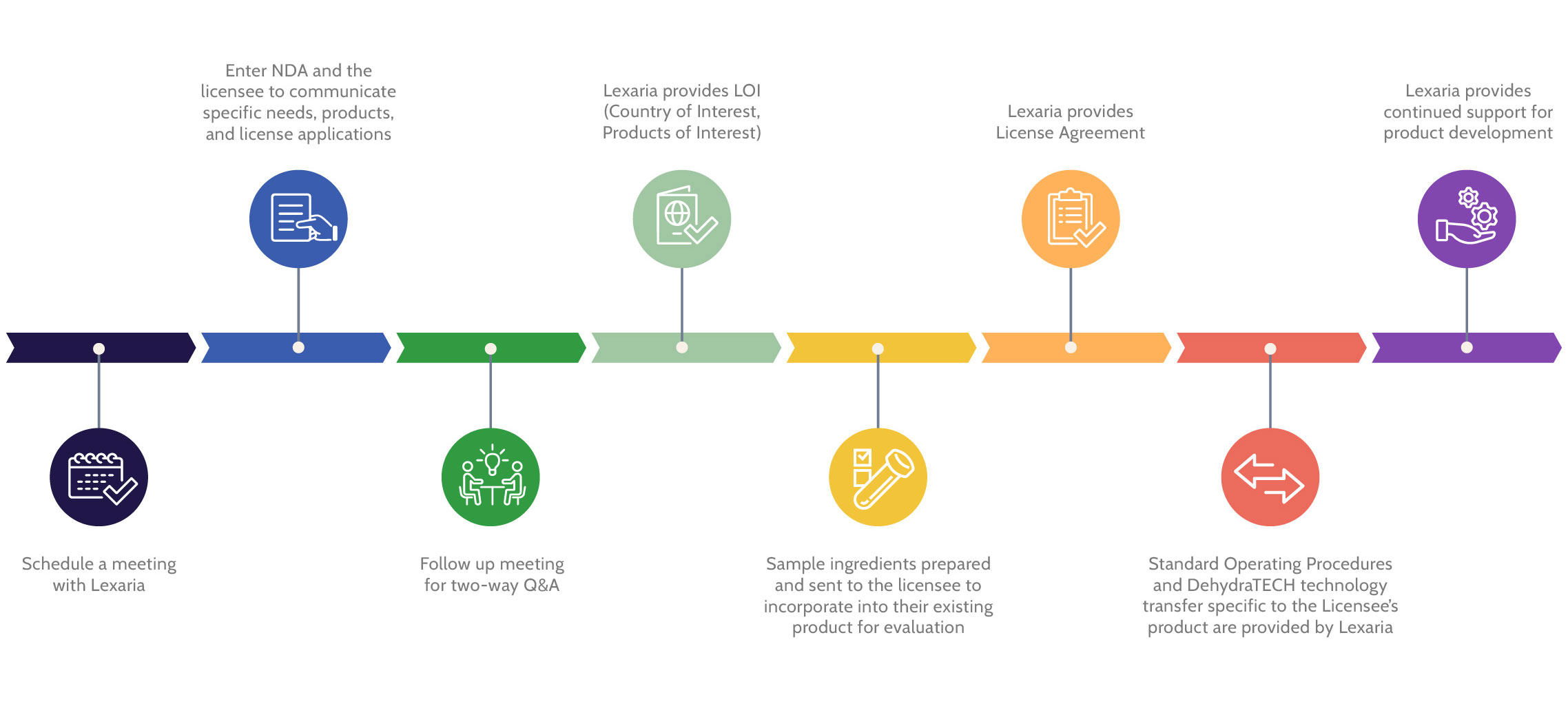
Lexaria has demonstrated its ability to enter formal agreements with Fortune 500 companies, and we will continue to foster new partnerships.
We are actively developing lead product pipeline candidates in the areas of:
- Hypertension and potentially heart disease
- GLP-1 drugs/diabetes and weight loss
- Antiviral drugs
Lexaria engages strategic partnerships with third party companies interested in exploring formulation opportunities with their specific APIs of interest.
We also out-license our technology in exchange for up-front fees, milestone payments and/or royalty payments, and we offer dietary supplement GMP-grade intermediate formulations to corporate clients on a toll processing basis.